Now Available: Prospera™ Heart for Pediatrics
Learn MoreNow Available: Prospera™ Heart for Pediatrics
Learn MoreNow Available! Prospera™ Heart with DQS
Extensive history and experience with cell-free DNA across industries powers new innovative features including a proprietary technique to estimate both the quantity and the fraction (dd-cfDNA %) of donor derived cell-free DNA in a single blood test.
Introducing Donor Quantity Score (DQS) to the Prospera™ Heart test for a clearer picture of rejection risk
Traditional dd-cfDNA tests
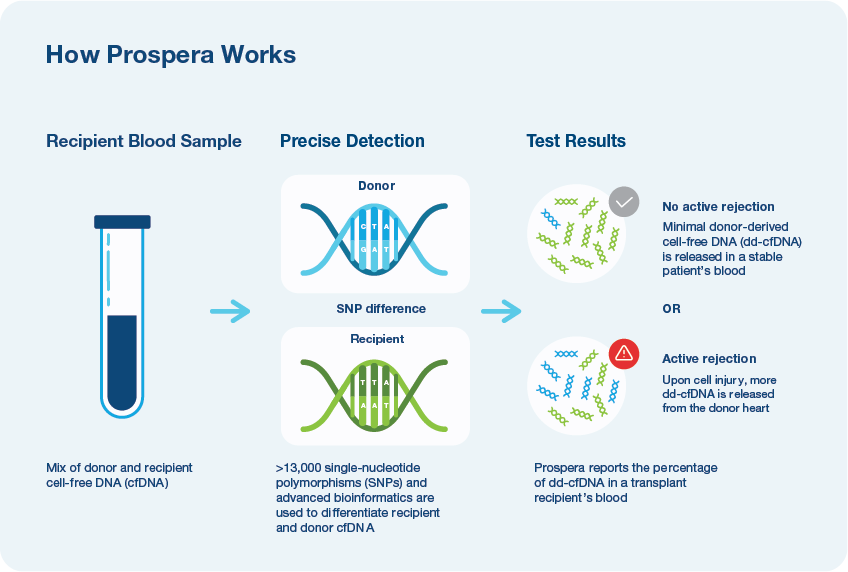
- Detect DNA in the blood from the donor heart and reports this as a fraction of the total cell-free DNA in the blood.
- The fraction of dd-cfDNA may be impacted when the amount of total cell-free DNA is atypically high or low.
DQS is the next evolution of dd-cfDNA results
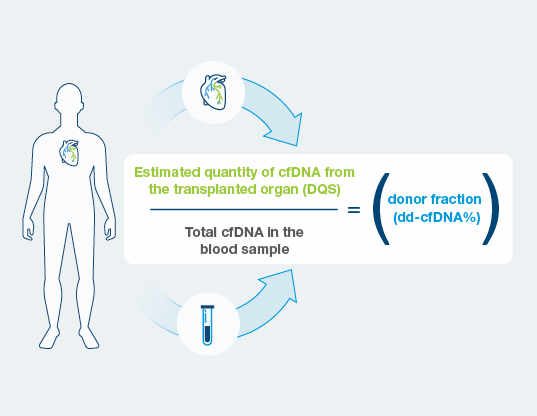
- DQS is the estimated quantity of cell-free DNA coming from the donated heart.
- Unlike traditional dd-cfDNA tests, DQS is independent of fluctuations in levels of total cell-free DNA in the blood.
Prospera™ Heart with DQS – the next generation
- Developed by Natera, a leader in cell-free DNA (cfDNA) with a trusted legacy in fetal monitoring, oncology and organ health
- Demonstrated in over 10 million tests1
- Utilizes over 13,000 pan-ethnic SNPs and advanced bioinformatics2
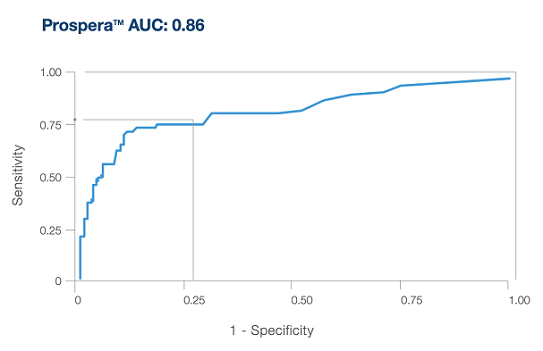
- Only Prospera™ Heart with DQS provides two donor derived cell-free DNA metrics and has been shown to improve accuracy in screening for acute cellular rejection (ACR) and antibody mediated rejection (AMR).3
Brief video to learn more about Prospera™ Heart with DQS
ISHLT Webinar Presented by Natera
Two Thresholds, One Powerful Solution: Combining the Quantity and Fraction of dd-cfDNA in a Single Blood Test to Improve the Accuracy of Noninvasive Rejection Screening Post Heart Transplantation
Duration: 60 minutes
Noninvasive monitoring for acute rejection with donor derived cell free DNA (dd-cfDNA) is a proven alternative to invasive surveillance biopsies. Until recently, dd-cfDNA tests have only reported dd-cfDNA as a fraction of total cell free DNA (dd-cfDNA %) in the circulation. This fraction can be confounded by fluctuations in the amount of total cell free DNA, sometimes caused by factors unrelated to the health of the allograft, including infection, surgery, or chemotherapy. Prospera™ Heart now incorporates a second metric – the Donor Quantity Score (DQS) – which is the estimated quantity of cell-free DNA coming from the donated heart, and is independent of fluctuations in levels of total cell-free DNA.
This webinar explores clinical implications of adding a second threshold to noninvasive monitoring for rejection. Dr. Bunnapradist, a transplant nephrologist from UCLA, provides a unique perspective as a long time user of this technology. Additionally, panelists Drs. Lampert and Rao discuss their first hand experience with Prospera™ with DQS in heart transplantation. Hear their early accounts including relatable case reviews.
Watch now to learn how the addition of DQS significantly reduces the rate of false positive results and improves the accuracy of detecting both acute cellular rejection (ACR) and antibody mediated rejection (AMR).

“There is a need for a sensitive, noninvasive surveillance tool for early detection of transplant injury to reduce frequency of biopsy in heart transplantation.”6
Why Prospera?
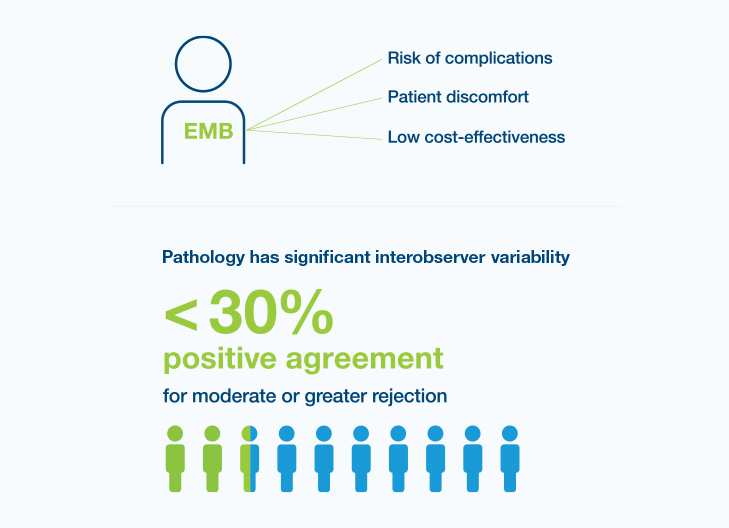
The current surveillance landscape has limitations and the management of heart transplant recipients is complex and challenging
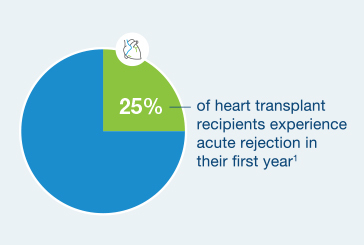
25% of heart transplant recipients experience acute rejection in their first year4
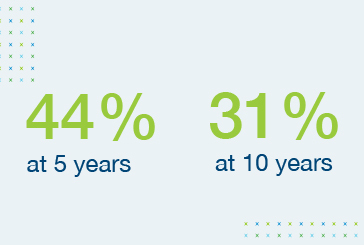
Survival rates of heart transplant recipients show little improvement over time5

Though endomyocardial biopsy (EMB) is the gold standard in surveillance for acute rejection, it has recognized challenges
Precision optimized for enhanced performance
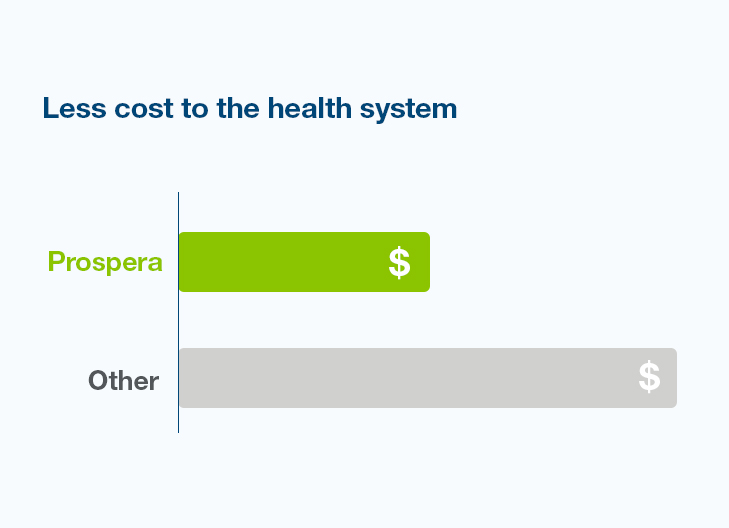
Reduce the number of surveillance biopsies by using Prospera as your first-line surveillance test
Patients first, partners always
Natera’s suite of solutions allows for streamlined process integration for your center
Total EMRSync
A complete, secure bi-directional data and workflow interface with Epic and Cerner systems
- Allows for easy ordering and delivery of results

ProsperaLink Program
A concierge team of clinical experts including a medical science liaison, nurse coordinator, & patient coordinator to help patients stay updated on blood draws, compliance plans and results.

Financial Support Program
- Natera welcomes all insurances, and our goal is to make the process easy and transparent for patients
- In the rare event your patient has financial responsibility, Natera offers flexible financial assistance programs and will work closely with your patient to ensure there is no hardship on them or their family.
Find out more about Prospera for heart transplant recipients
References
1Natera Inc. Natera validation data: manuscript submitted. Data on file.
2Sigdel TK, Archila FA, Constantin T, et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med. 2018 (per published article);8(1):19 doi:10.3390/jcm8010019
3Kim, P et al. A Two-Threshold dd-cfDNA Algorithm for Detection of Rejection After Heart Transplant. J Heart Lung Transplant; 2024; 43(4):S205-S206
4U.S. Department of Health & Human Services: Health Resources and Services Administration. Scientific Registry of Transplant Recipients (SRTR): Organ Procurement and Transplantation Network (OPTN)/SRTR 2019 Annual Data Report: Heart. Available at: https://srtr.transplant.hrsa.gov/annual_reports/2019/Heart. aspx#HR_tx_adult_inc_AR_age_b64. Accessed June 1, 2021.
5Altug Y, Liang N, Ram R, et al. Analytical validation of a single-nucleotide polymorphism-based donor-derived cell-free DNA assay for detecting rejection in kidney transplant patients. Transplantation. 2019;103(12):2657-2665. doi:10.1097/TP.0000000000002665
6Toyoda Y, Toyoda Y. Heart-lung transplantation: adult indications and outcomes. J Thorac Dis. 2014;6(8):1138-1142. doi:10.3978/j.issn.2072-1439.2014.06.01
7Kim PJ, Olymbios M, Siu A, et al. A novel donor-derived cell-free DNA assay for the detection of acute rejection in heart transplantation. J Heart Lung Transplant. 2022; doi:10.1016/j.healun.2022.04.002