New Medicare Coverage: Signatera in NSCLC
Learn MoreNew Medicare Coverage: Signatera in NSCLC
Learn MoreMonitor Lung Cancer with Signatera™
Stay proactive with personalized cancer monitoring using Signatera™. This ctDNA test detects molecular residual disease (MRD) for early intervention, helping to provide peace of mind and guiding your treatment decisions. Whether you’ve undergone surgery, radiation, or other treatments, Signatera™ helps monitor your cancer status. With regular serial testing, lung cancer survivors can catch molecular recurrence early, before imaging detects changes, allowing for timely action.
For lung cancer patients undergoing treatments like surgery or immunotherapy, Signatera™ provides personalized monitoring to detect molecular changes and track your response to treatment.
Learn how Signatera™ can empower you to manage your lung cancer journey effectively.
Why Signatera™ for Lung Cancer?
For lung cancer survivors, monitoring your cancer is crucial. Signatera™’s personalized ctDNA test detects cancer recurrence earlier than traditional scans, potentially allowing for timely intervention. With frequent serial testing, you can gain peace of mind knowing that signs of molecular recurrence can be caught earlier. Whether you’ve completed surgery, radiation, or immunotherapy, Signatera™ provides real-time insight into your cancer status, track your response to treatment and monitor for recurrence, helping you and your oncologist make informed decisions about your care.
Immunotherapy (IO) Monitoring with Signatera™
f you’re receiving immunotherapy for lung cancer, Signatera™ can help monitor your response to treatment. By tracking ctDNA levels, Signatera™ offers insights into how well your body is responding to immunotherapy, helping your oncologist adjust treatment strategies when necessary. Early detection of molecular changes allows for more personalized care throughout your cancer journey.
How does Signatera™ testing work?
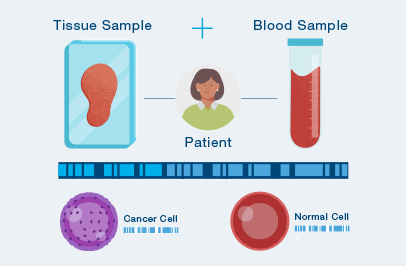
The first time your doctor orders Signatera™, a one-time tissue sample and a blood sample are needed to build your unique test.
Natera will work with your cancer care team to procure your tumor tissue from a prior procedure or surgery.

After your test is built, you only need to provide a blood sample each time your doctor orders Signatera™.
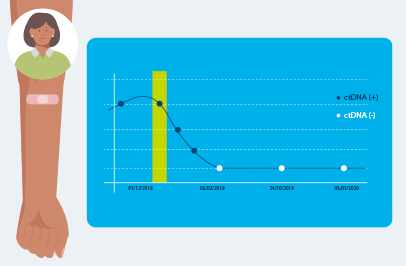
Repeated Signatera™ testing can show changes in your ctDNA levels, helping your doctor understand if your cancer is shrinking, growing, or coming back.
Genetic Testing for Lung Cancer Patients
In addition to personalized cancer monitoring with Signatera™, genetic testing plays a vital role in lung cancer care. The Altera™ Tumor Profiling Test can help determine if targeted or immunotherapy will be effective by analyzing genes like EGFR and ALK. This personalized approach is especially important for non-small cell lung cancer (NSCLC) patients.
By combining Signatera™ with Altera™, lung cancer patients can personalize both their current therapies and future care, ensuring their treatment is tailored to their genetic profile.

Be Your Own Best Advocate
Ask your doctor about Signatera™
If you or your loved one have been diagnosed with lung cancer, you likely have many questions including “What do I do next?” When used alongside other tests, Signatera™ can help answer critical questions to guide your care:
- Is there cancer left after my surgery or radiation?1-3
- Is my cancer coming back?1-2
- Is my immunotherapy working?3
Gain Reassurance With Signatera™
After JoAnn was diagnosed with lung cancer, she learned how Signatera™ could help her doctor detect potential disease worsening ahead of scans. As part of her cancer monitoring plan, she received Signatera™ testing at regular intervals, helping her and her doctor make critical decisions.
Learn how Signatera™ helped JoAnn and her family stay confident and positive throughout her treatment journey.
“Signatera™ is a great warning system that something is going on. From there, you figure out what it is. Signatera™ does empower me to be my own best advocate with my oncologists.” – JoAnn, living with lung cancer
Personalize Your Lung Cancer Care

Understand Your Risk
Using Signatera™ after surgery or radiation can predict your risk of lung cancer recurrence and may identify whether you could benefit from additional treatment.1-2

Detect Recurrence Early
Signatera™ can provide an early alert that your cancer is coming back, 4-5 months before CT scans.1-2

Know If Immunotherapy Is Working
As early as 6 weeks into treatment, Signatera™ can determine if your cancer is responding to immunotherapy.3
Access Testing
Billing
- Signatera™ is covered by Medicare for stage I-III non-small cell lung cancer (NSCLC) in the surveillance setting, as well as for any patient being treated with immunotherapy.
- We welcome all insurance plans. Please refer to our list of in-network plans that we participate with, or call your insurance provider.
- We offer financial assistance programs for those that qualify.
Your Top Questions
- Find answers to your questions about eligibility, results, ordering, and more.
- Don’t see your question? Contact us here.
Learn More

Coverage and Billing Guide
Read our billing guide for information about understanding Natera’s testing bill, including coverage, financial assistance programs, and billing support services.

GO2 Foundation for Lung Cancer
GO2 Foundation for Lung Cancer transforms survivorship as the world’s leading organization dedicated to saving, extending, and improving the lives of those vulnerable, at risk and diagnosed with lung cancer. We work to change the reality of living with lung cancer by ending stigma, increasing public and private research funding, and ensuring access to care.

Lungevity
LUNGevity is focused on improving outcomes for people with lung cancer through research into early detection and more effective treatments, policy initiatives, education, community, and support for patients and caregivers.
Is Signatera™ right for you?
References
1 Abbosh C, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446-451. https://doi.org/10.1038/nature22364
2 Lebow ES, et al. Minimal residual disease (MRD) detection by ctDNA in relation to radiographic disease progression in patients with stage I-III non-small cell lung cancer (NSCLC) treated with definitive radiation therapy. Journal of Clinical Oncology 2022 40:16_suppl, 8540-8540. https://doi.org/10.1200/JCO.2022.40.16_suppl.8540
3 Bratman SV, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873-881. https://doi.org/10.1038/s43018-020-0096-5