Monitor Your Gynecologic Cancer
The Signatera™ Residual Disease Test is a custom-built blood test for people who have been diagnosed with gynecologic cancers like ovarian or uterine cancer. Signatera™ can detect molecular residual disease (MRD) in the form of circulating tumor DNA—small fragments of DNA released by cancer cells.
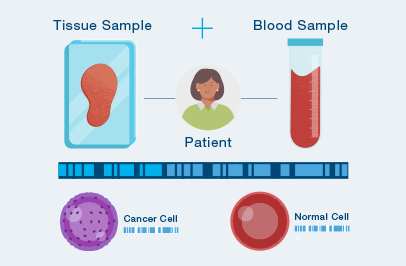
The first time your doctor orders Signatera™, a one-time tissue sample and a blood sample are needed to build your unique test.

After your test is built, you only need to provide a blood sample each time your doctor orders Signatera™.
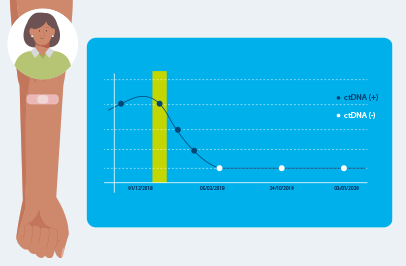
Repeated Signatera™ testing can show changes in your ctDNA levels, helping your doctor understand if your cancer is shrinking, growing, coming back, or responding to immunotherapy.

Get Early Information to Help Inform Your Care
When used alongside standard tests recommended by your doctor, Signatera™ can help find answers to crucial questions:
- Is your cancer likely to come back?1
Signatera™ may provide additional information when other tests are unclear.
Signatera™ can help detect cancer recurrence sooner than imaging and more accurately than traditional blood tumor markers for gynecologic cancers such as CA-125.1 - Is your treatment working?2
As early as six weeks into your immunotherapy treatment, Signatera™ can help you and your doctor understand if you are responding to your therapy.2
Take Action Sooner
Anne was receiving Signatera™ testing and CA-125 at regular intervals to monitor for recurrence of her ovarian cancer. When her Signatera™ test showed she was positive for ctDNA, her doctor ordered a scan, which confirmed her cancer was coming back.
Anne underwent additional treatment. Now, she continues to use Signatera™ alongside other tests to monitor how well she is responding to her maintenance therapy. Her Signatera™ results show that she is negative for ctDNA, helping her feel more confident that she does not have detectable cancer.

Inform Care Decisions Across Your Treatment Journey

During Treatment
Receiving Signatera™ testing over time can help monitor your response to treatment, like immunotherapy, by assessing changes in your ctDNA levels.2

After Treatment
SIgnatera™ testing may detect recurrence before it is visible on traditional imaging tests like CT scans, helping you and your doctor decide if you need to take action.2
Common Testing Questions
Medicare Coverage
- Signatera™ is covered by Medicare for patients with stage II-IV ovarian, fallopian tube, or primary peritoneal cancer.
- We welcome all insurance plans. Please refer to our list of in-network plans that we participate with, or call your insurance provider.
- We offer financial assistance programs for eligible people.
More Information
- Find answers to your questions about eligibility, results, ordering, and more.
- Don’t see your question? Contact us here.
Learn More

Learn how adding Signatera™ testing to your cancer monitoring plan can help you get important information, earlier.

Learn how Signatera™ can help monitor treatment response in people with gynecologic cancers.
Is Signatera™ right for you?
References
1Chapman J, et al. Circulating tumor DNA predicts disease recurrence in ovarian cancer patients. Poster presented at the American Association of Cancer Research annual meeting; April 10-15, 2021; Virtual Meeting. Abstract 552.
2Bratman SV, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873-881. https://doi.org/10.1038/s43018-020-0096-5
