Inform Clinical Challenges in Colorectal Cancer
Identify High Risk Patients
80%
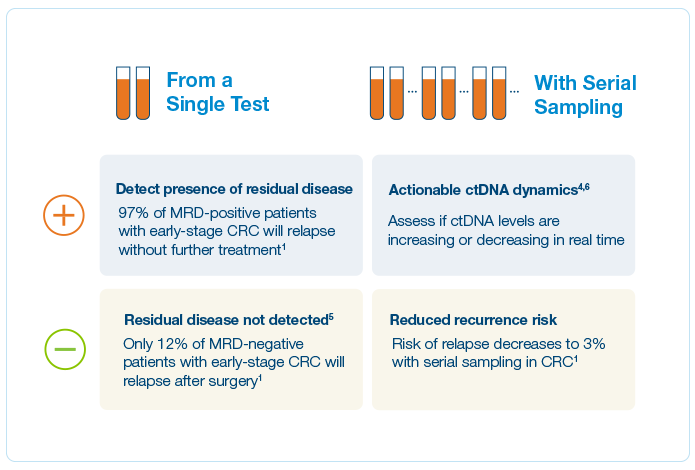
Of CRC patients are cured by surgery alone — knowing which patients would benefit from ACT is often unclear1
Predict Overall Survival
36-month OS
96%
in ctDNA negative patients vs. 71.8% in ctDNA positive patients2
ctDNA clearance and survival
100%
MRD-positive patients who achieved sustained clearance had 100% OS at 24 months2
Get Started
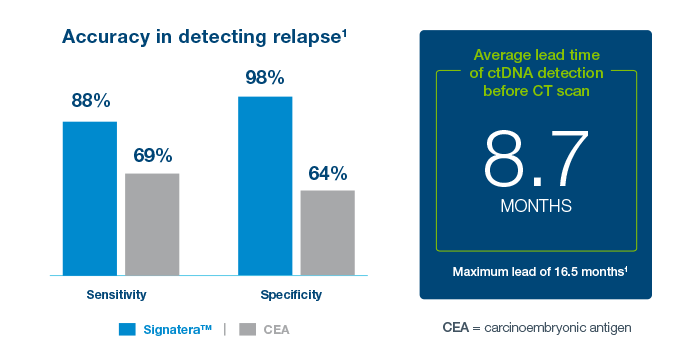
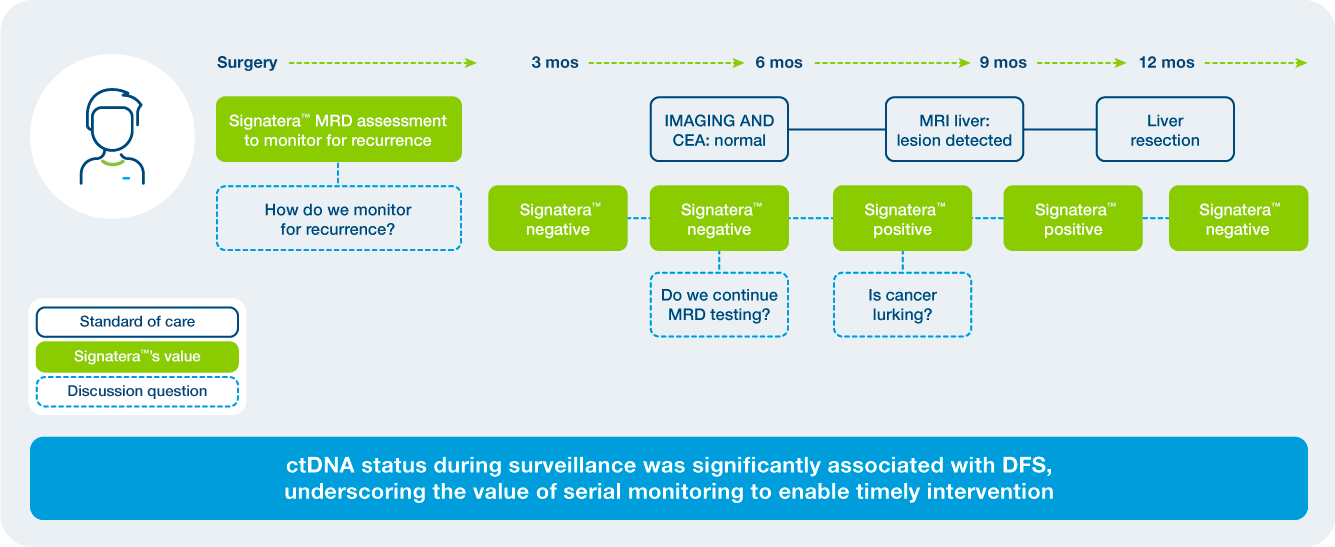
Clinical Application: What’s the value of serial testing in the surveillance setting?
Predict Adjuvant Treatment Benefit
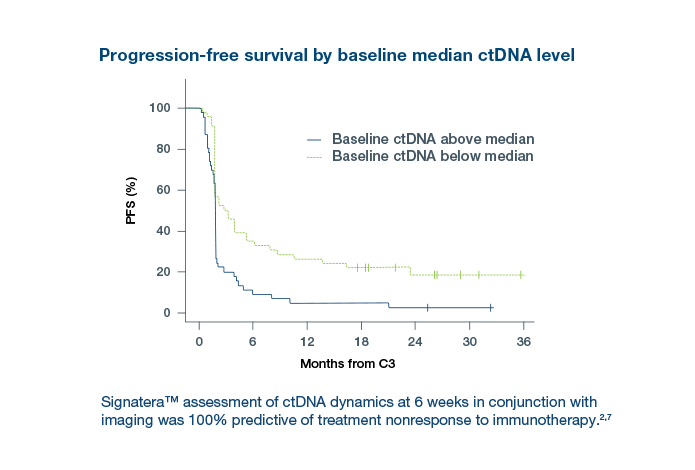
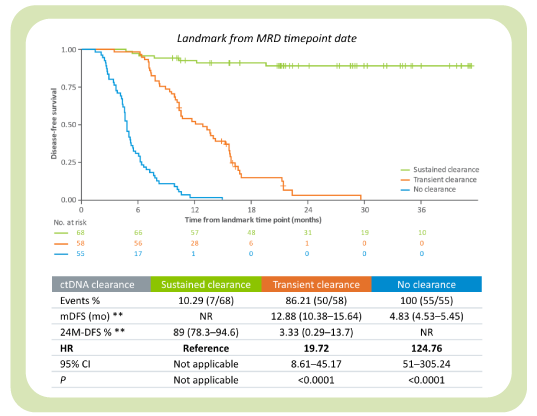
The latest GALAXY findings2 from the CIRCULATE-Japan trial analyzes >2,240 patients with stage II– IV colorectal cancer and includes 36M-DFS and 24M- OS data demonstrating the importance of post-surgical ctDNA analysis and its association with long term survival:
- Reinforces Signatera™’s ability to predict adjuvant chemotherapy (ACT) benefit in resectable CRC
- Signatera™’s ability to identify sustained ctDNA clearance is an indicator of a superior survival benefit derived from ACT, compared to patients who did not achieve ctDNA clearance
- Patients who clear their ctDNA and remain Signatera™-negative have superior DFS and OS, compared to those with transient clearance converting back positive and those with no ctDNA clearance
Powering Personalized Decisions in Early and Late Stage CRC
Discover the Colorectal Cancer Data
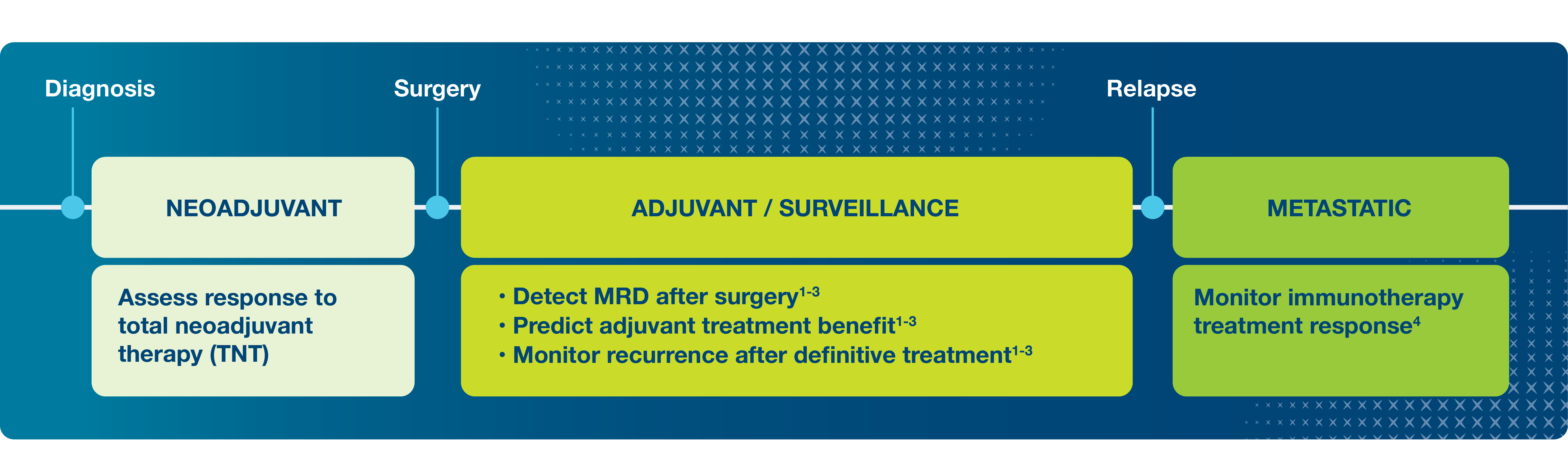
- Signatera™ MRD testing in colorectal cancer is validated across treatment settings.
-
Neoadjuvant Response Monitoring
- Monitor neoadjuvant response with serial Signatera™ testing.
- Identify low risk patients who are ctDNA-negative to potentially support a nonsurgical “watch and wait” approach.
-
Post-Surgical MRD Assessment
-
Recurrence Monitoring
-
ctDNA clearance
Enabling time to process and make informed plans
“Signatera fired the warning shot that something was not right. Together with my HCP we had the opportunity to take the time to consider the options. She put the choice on me on what to do with a positive test result and it enabled me to choose to take a more aggressive approach.”
Keith, CRC patient
Covered by Medicare for multiple solid tumor indications
Learn More About Signatera™ in Colorectal Cancer
Ready to try Signatera™ for your colorectal cancer patients?
References
1Reinert T, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124-113. https://doi.org/10.1001/jamaoncol.2019.0528
2Kotani D. et al., Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer, Nature Medicine v29 Issue 1 Jan 2023
3Kotani D. et al., Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer, Nature Medicine v29 Issue 1 Jan 2023
4Bratman SV, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873-881. https://doi.org/10.1038/s43018-020-0096-5
5Loupakis F, et al. Detection of molecular residual disease using personalized circulating tumor DNA assay in patients with colorectal cancer undergoing resection of metastases. JCO Precis. Oncol. 2021(5):1166-1177. https://doi.org/10.1200/PO.21.00101
6Abbosh C, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446-451. https://doi.org/10.1038/nature22364
7Sinicrope FA, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–875. https://doi.org/10.1093/jnci/djr153
8Aoyama T, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Med. 2017;6(7):1573–1580. https://doi.org/10.1002/cam4.1126
9Yothers G, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol. 2013;31(36):4512-4519. https://doi.org/10.1200/JCO.2012.47.3116
10Corcoran RB, et al. Applications of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754-1765. https://doi.org/10.1056/NEJMra1706174