CGA-IGC webinar
GI-cancer case studies:
Utilizing oncologist-initiated testing and the new Natera Hereditary Cancer Alert Program
60 minute webinar
Featured Webinar
[GI-Cancer Case Studies] Capture More Patients & Families Who Can Benefit from Hereditary Cancer Testing: Utilizing the Natera Hereditary Cancer Alert Program and Oncologist Initiated Testing
Join Dr. Shruti Singh, Medical Oncologist / Hematologist at Northwest Cancer Centers, Natera and CGA IGC for a 60-minute webinar to discuss how providers in the Oncology clinic can initiate hereditary cancer testing through the new Natera Hereditary Cancer Alert Program to capture more patients who can benefit from genetic information.
Webinar Objectives:
- Explore how to implement oncologist / clinician initiated hereditary cancer testing into workflows and the resulting impact on patient outcomes
- Learn how the new Natera Hereditary Cancer Alert Program (HCAP) works to help identify more patients who meet testing guidelines in Oncology clinics
Closing the Hereditary Cancer Testing Gap
Natera is dedicated to ensuring genetic information is accessible to all.
Many affected patients who could benefit from hereditary cancer testing are not tested. A study of >1.36M patients diagnosed with cancer between 2013-2019 uncovered that only 6.8% had hereditary cancer testing within 2 years of their diagnosis.1
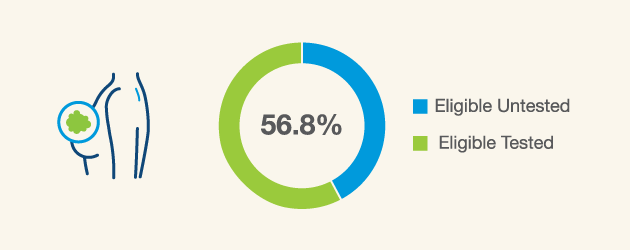
Breast cancer patients ≤50 years of age and patients with TNBC are eligible for hereditary cancer testing,2 however just a little over half are actually receiving it3
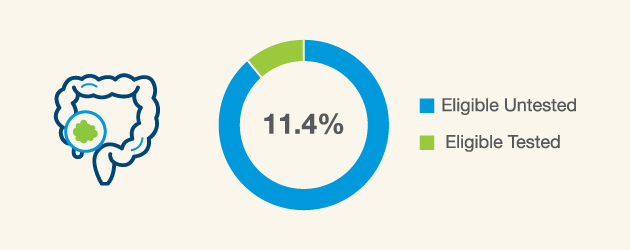
Many newly diagnosed colorectal cancer (CRC) patients are potentially eligible for hereditary cancer testing4 however only 11.4% are receiving this type of testing5
The Empower™ Hereditary Cancer Test is Designed with Your Practice in Mind
Five panel options with up to 81 genes across 12+ common hereditary cancer types, and customizable gene panels with 190+ gene options. Genes can be selected individually or added by selecting a particular organ system of interest.

Hereditary breast and ovarian cancer syndrome
Genes: BRCA1, BRCA2
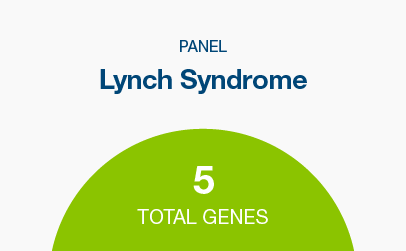
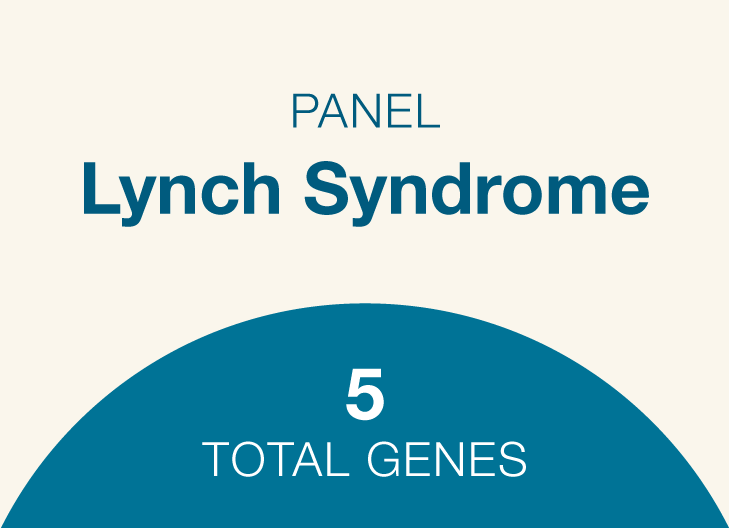
Lynch syndrome
Genes: MLH1, MSH2, MSH6, PMS2, EPCAM
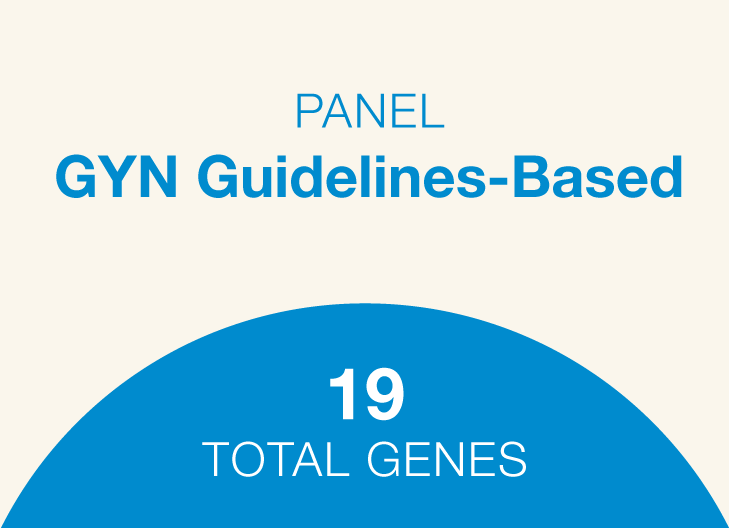
Breast, ovarian, endometrial cancers and Lynch syndrome genes
Commonly screened-for hereditary cancer genes, plus genes with emerging evidence of elevated cancer risks
Genes: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53

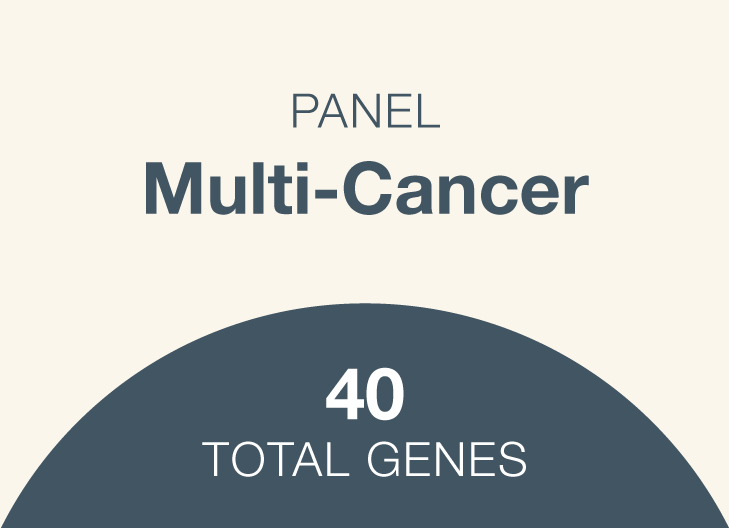
Most commonly screened-for hereditary cancer genes across eight cancer types
Genes: APC, ATM, AXIN2, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, GALNT12, GREM1, HOXB13, MEN1, MITF, MLH1, MSH2, MSH3, MSH6, MUTYH, NBN, NF1, NTHL1, PALB2, PMS2, POLD1, POLE, PTEN, RAD51C, RAD51D, RNF43, RPS20, SMAD4, STK11, TP53, VHL
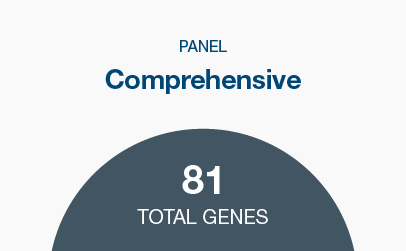
Commonly screened-for hereditary cancer genes, plus genes with emerging evidence of elevated cancer risks
Genes: AIP, ALK, APC, ATM, AXIN2, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDC73, CDH1, CDK4, CDKN1B, CDKN1C, CDKN2A, CEBPA, CHEK2, CYLD, DDX41, DICER1, EGFR, EPCAM, EXT1, EXT2, FH, FLCN, GATA2, GREM1, HOXB13, KIT, LZTR1, MAX, MEN1, MET, MITF, MLH1, MSH2, MSH3, MSH6, MUTYH, NBN, NF1, NF2, NTHL1, PALB2, PDGFRA, PHOX2B, PMS2, POLD1, POLE, POT1, PRKAR1A, PTCH1, PTEN, RAD51C, RAD51D, RB1, RET, RHBDF2, RUNX1, SDHA, SDHAF2, SDHB, SDHC, SDHD, SMAD4, SMARCA4, SMARCB1, SMARCE1, STK11, SUFU, TERC, TERT, TMEM127, TP53, TSC1, TSC2, VHL, WT1
*Breast STAT panel available with 10 breast cancer genes reported within 5-7 calendar days + 71 additional genes reported within 2 weeks. Breast STAT genes include ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, PALB2, PTEN, STK11 and TP53.
“Consider germline MGPT evaluation for all individuals with a personal history of colorectal cancer or endometrial cancer at any age.”
A Responsibility to Patients
Natera’s Hereditary Cancer Alert Program (HCAP) helps identify more patients who could benefit from hereditary cancer testing.
The Alert Program increased patients tested by 200% in a pilot with cancer centers, supporting precision patient management options.
Natera supports one of the largest cancer patient cohorts in the U.S., reaching hundreds of thousands of patients with multiple touchpoints annually with the Signatera™ Residual Disease Test (MRD). Natera created the Hereditary Cancer Alert Program (HCAP) to automatically flag Signatera™ patients that meet testing guidelines via an opt-in monthly report sent to the referring physician and genetic counseling staff.
The Empower™ Hereditary Cancer Test: Customizable Gene Panel Options
- Five panel options with up to 81 genes across 12+ common hereditary cancer types, and customizable gene panels with 190+ gene options. Genes can be selected individually or added by selecting a particular organ system of interest.
-
BRCA1 and BRCA2
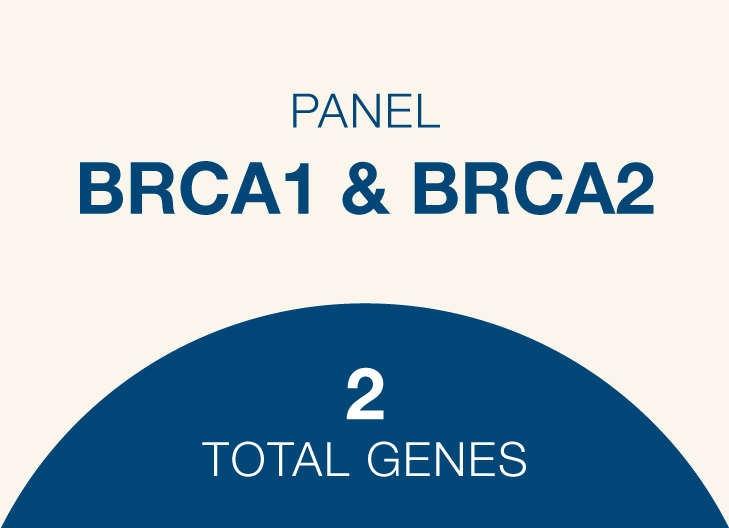 Genes: BRCA1, BRCA2
Genes: BRCA1, BRCA2 -
Lynch syndrome
-
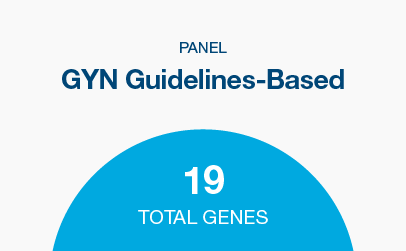
GYN Guidelines-Based
-
Multi-Cancer
-
Comprehensive
- *Breast STAT panel available with 10 breast cancer genes reported within 5-7 calendar days + 71 additional genes reported within 2 weeks. Breast STAT genes include ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, PALB2, PTEN, STK11 and TP53.
View and create your own test panel
- Choose from over 190 genes associated with 12+ organ systems
- Add or remove single genes to build test panels with a single click
- Customize and save panels to use in the future
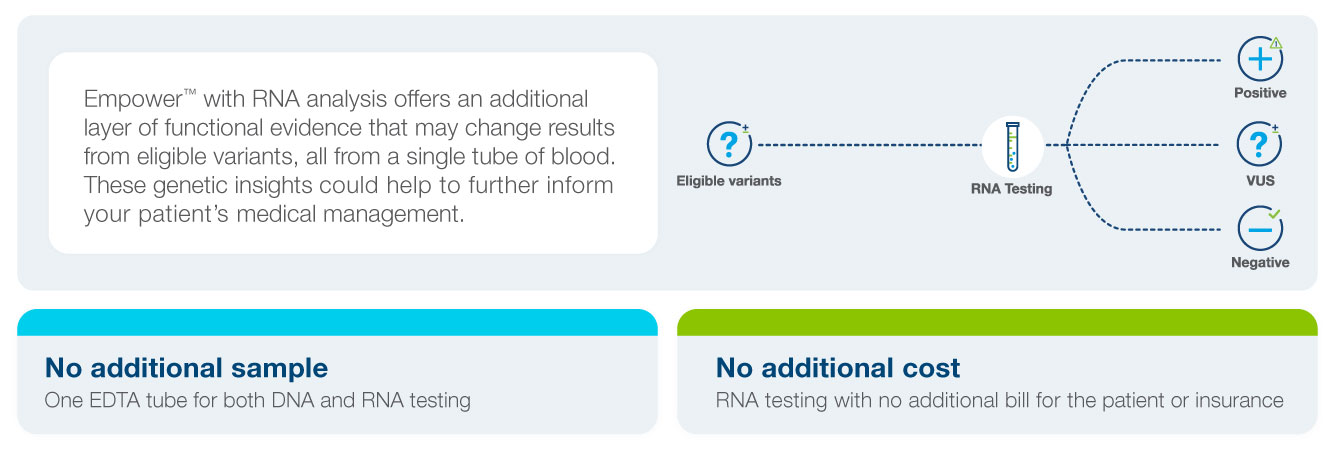
RNA Analysis for Empower™ – No Additional Sample Required
RNA analysis for Empower™ provides even more health insights, with no additional sample required. RNA testing can help to improve detection and classification of certain variants that fall within splice site regions.
If there is evidence that a variant detected may impact splicing, RNA analysis is completed*. An updated report is automatically issued to you and your patient if a variant is reclassified with RNA analysis.
Testing includes VUS splice sites across 54 high and moderate penetrance genes.
*RNA analysis is performed if select criteria are met, including if blood is the specimen type and the splice site variant is reported within 21 days of sample collection.

Actionable Reports Help Guide Precision Patient Management
- Identify patients eligible for treatments, such as PARP inhibitors
- Predict response to therapies, such as cisplatin chemotherapy
- Inform surgical decisions
- Illuminate risk for the development of new cancers to offer proactive screening and prevention
- Identify the risk of mutation being present in family members to help them proactive manage hereditary cancer risk
Same-Size Panel Family Testing Program – No Additional Charge
Testing for all blood relatives of patients with a positive result is available at no additional charge.* The test can be ordered by the provider with a copy of the original family member’s test result. Natera offers complimentary panels of the same size to previous relative’s test order.

Commitment to Affordability
Natera is proud to be an in-network provider with most health plans, including Anthem, Cigna, and United Healthcare.
Natera also offers self-pay pricing, interest-free payment plans and compassionate care options.
Receive a personalized cost estimate for Empower™ by texting 1-650-210-7046 or emailing estimate@natera.com.
Support Every Step of the Way
- Complimentary pre- and post- test genetic information sessions
- Flexible phlebotomy options with in-lab and end-to-end remote testing available
- Patient friendly reports and supplements
- A range of ordering options and EMR integration offered
- Spectrum Advantage Program and Horizon Partner Auto-Enroll
- Texting Program
NEVA, Natera Educational Virtual Assistant
- Streamlined family cancer history intake
- Interactive results delivery and genetic education, available 24/7


Patient management recommendations based on medical guidelines
- Detect cancer at its earliest, most treatable stage
- Identify risk-reducing medications and surgeries
- Inform surgical and therapeutic decisions following a cancer diagnosis
- Notify family members to help them proactively manage hereditary cancer risk
Women’s Health & Imaging Centers
Dr. Amber Shamburger
OB GYN
“We wanted to catch people at every point of screening across the hospital – annual mammography, pap smear, colonoscopy, low dose CT, etc. A lot of people try to stay away from hospitals and do not see specialists. How do you catch all these people? Every patient gets a hereditary cancer risk screener.”
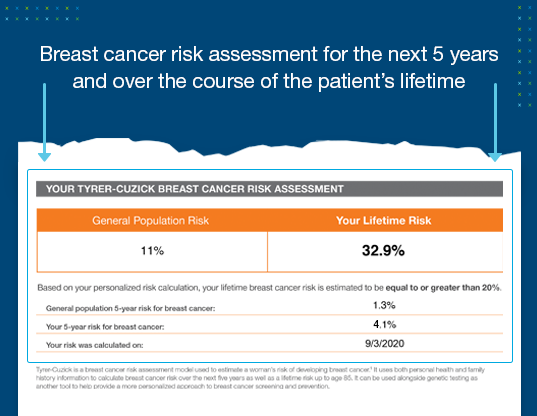
Tyrer-Cuzick results for a comprehensive breast cancer risk assessment
- Patients without a breast cancer-related gene mutation may still have increased risk for breast cancer based on their family cancer history and estrogen exposure over their lifetime.
- Patients with ≥20% remaining lifetime breast cancer risk qualify for insurance coverage for preventive screenings including annual breast MRI surveillance in addition to an annual mammogram.
- Tyrer-Cuzick evaluations are recommended by medical guidelines to calculate a woman’s breast cancer risk in the next 5 years and over the course of her lifetime.
- Empower reports offer clear medical management information based on genetic and family history risk.

Natera is committed to patient affordability
Natera is proud to be an in-network provider with most health plans, including Anthem, Cigna, and UnitedHealthcare.
For patients without adequate insurance coverage, Natera also offers self-pay pricing and compassionate care options.
Support every step of the way
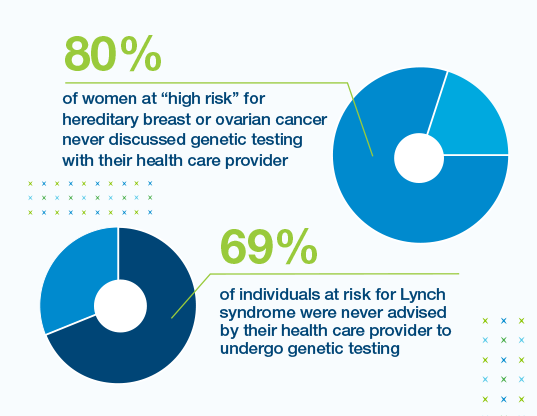
Many patients at high risk for hereditary cancer are not tested
Disclaimers : Bellcross CA, Peipins LA, McCarty FA, Rodriguez JL, et al. Genet Med. Characteristics associated with genetic counseling referral and BRCA1/2 testing among women in a large integrated health system. 2015 Jan;17(1):43–50. Patel SG, Ahnen DJ, Kinney AY, et al. Am J Gastroenterol. Knowledge and uptake of genetic counseling and colonoscopic screening among individuals at increased risk for lynch syndrome and their endoscopists from the family health promotion project. 2016 Feb;111(2):285-93.
When to consider hereditary cancer testing
Personal or family history of cancer at age 50 or younger
Personal or family history of ovarian, male breast or pancreatic cancer
Multiple cancers or tumors on the same side of the family
Ashkenazi ancestry
Your patient is concerned about other familial cancer patterns
Is Empower™ right for you?
We’re here to help you find out
References
1Cancer risk estimates for a positive result are typically based on individuals with a family or personal history of cancer. NCCN Clinical Practice Guidelines in Oncology Genetic/Familial High Risk Assessment: Breast, Ovarian and Pancreatic v2.2021
2Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. JCO. 2017 Dec 1; 35:3800-38063.
References
1Kurian, A., Abrahamse, P., Furgal, A., et al. Germline Genetic Testing After Cancer Diagnosis. JAMA, E1–E9(2023). https://doi.org/10.1001/jama.2023.9526
2NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic.
3Allison W. Kurian et al., Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. JCO 37, 1305-1315(2019). DOI:10.1200/JCO.18.01854
4NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2022 - June 8, 2022.
5Moretz C, Byfield SD, Hatchell KE, et al. Comparison of Germline Genetic Testing Before and After a Medical Policy Covering Universal Testing Among Patients With Colorectal Cancer. JAMA Netw Open. 2022;5(10):e2238167. doi:10.1001/jamanetworkopen.2022.38167