Now Available: Prospera™ Heart for Pediatrics
Proven cell-free DNA technology for noninvasive transplant rejection monitoring in pediatric heart transplantation, ages 7+
What is Prospera™ Heart?
Prospera™ Heart is a precise, noninvasive donor-derived cell-free DNA (dd-cfDNA) blood test that is optimized to assess the health of a transplanted heart. Prospera™ leverages Natera’s extensive experience with cell-free DNA to analyze the fraction of dd-cfDNA from the transplanted organ.
Confidently rule-out rejection with high sensitivity and negative predictive value (NPV) to avoid unnecessary and invasive endomyocardial biopsies (EMBs).1
Biopsy puts patients at risk
Endomyocardial biopsies (EMBs) are commonly used for allograft rejection monitoring after heart transplantation. However, EMBs have limitations that can be costly to the patient and may be interpreted differently by different pathologists. 2-7
Invasive & risky
- Need for general anesthesia
- Risk of infection
- Potential for tricuspid valve damage
- Vascular thrombosis
Inconvenient & costly
- Significant time and travel required
- Difficult for patients to manage as they transition to independent adulthood
- Patient discomfort and ongoing stress
Potential failures
- Inadequate tissue sampling
- High interobserver variability

Reduce invasive biopsies for kids
Unlike traditional biopsies, Prospera™ Heart provides early information about possible rejection without the need for anesthesia, or the risk and inconvenience of an invasive procedure.
How Prospera™ works
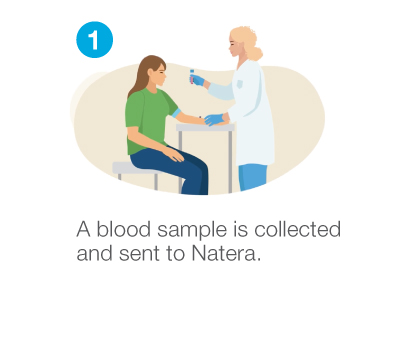
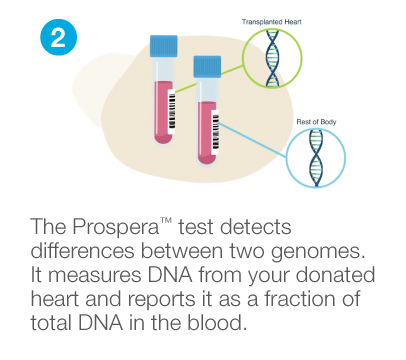
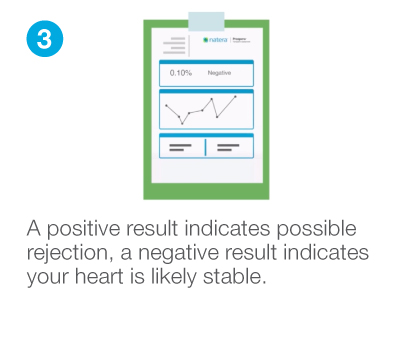
Highly accurate results
The DTRT-2 study adds to the growing body of evidence in support of using Prospera™ to confidently rule-out antibody mediated rejection (AMR) and acute cellular rejection (ACR) in heart transplant patients of all ages. 1
NPV*
(negative predictive value)
99%
AUC
(area under the curve)
0.83
DTRT-2 study set the optimal threshold cutoff at 0.275%.
*Rejection prevalence of 5%; rejection defined as ACR ≥2R and/or AMR ≥1
Perspective on Natera
“As the President of Transplant Families, I have witnessed firsthand how Natera has profoundly impacted families with pediatric transplant patients with their cutting-edge genetic testing and monitoring services which provide critical insights into post-transplant health and wellness, empowering families and their transplant team with timely, actionable information. This support is the pinnacle of patient centered care and significantly reduces the emotional and financial burdens on families. Natera’s commitment to precision medicine and compassionate service truly makes a difference in the lives of our youngest transplant recipients.””
Melissa McQueen
CEO/President Transplant Families and
Mother of heart recipient
Commitment to affordability
- We welcome all insurance plans.
- As part of our commitment to providing accessible testing, most Prospera™ patients have no out-of-pocket expenses. In the rare situation where there may be a bill due to co-pay or co-insurance, we will provide information on our financial assistance programs.
Easy, fast, and reliable testing
Create a schedule for easy and convenient blood draws to track dd-cfDNA levels over time for longitudinal surveillance data.
Draw as early as 28 days
post-transplant
No extra equipment needed
Simply draw and ship.
No spin down or freezing of sample
Fast turnaround
3-4 days
Find out more about Prospera™ Heart for pediatric heart transplant recipients
1Deshpande SR, Zangwill SD, Richmond ME, et al. Evaluating threshold for donor fraction cell-free DNA using clinically available assay for rejection in pediatric and adult heart transplantation. Pediatric Transplantation. 2024; 28:e14708. doi:10.1111/petr.14708
2Oh KT, et al. Protocol endomyocardial biopsy beyond 6 months – it is time to move on. Am J Transplant. 2021;21:825-9.
3Coutance G, et al. Clinical Prediction model for antibody-mediated rejection: A strategy to minimize surveillance endomyocardial biopsies after heart transplantation. Circ Heart Fail. 2022;15:e009923.
4From AM, et al. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011 Nov;86(11):1095-1102. doi: 10.4065/mcp.2011.0296.
5Bermpeis K, et al. Safety of Right and Left Ventricular Endomyocardial Biopsy in Heart Transplant and Cardiomyopathy Patients. JACC Heart Fail. 2022 Dec;10(12):963-973. doi: 10.1016/j.jchf.2022.08.005. Epub 2022 Oct 12.
6Kush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-six adult heart transplantation report – 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019 Oct;38(10):1056-1066. doi: 10.1016/j.healun.2019.08.004. Epub 2019 Aug 10.
7Shah KB, et al. Surveillance Endomyocardial Biopsy in the Modern Era Produces Low Diagnostic Yield for Cardiac Allograft Rejection. Transplantation. 2015 Aug;99(8):e75–e80. doi: 10.1097/TP.0000000000000615.