
Be part of the community helping to address colorectal cancer care
Do you want to be a part of something extraordinary? You and nearly one thousand other patients living with colorectal cancer have the opportunity to help the medical community to more deeply understand this disease. Learn more about our clinical study, BESPOKE CRC, which is currently enrolling patients living with colorectal cancer (CRC).


Enroll now to help the community learn more
The study will enroll at least one thousand patients. Natera and its collaborators will collect clinical utility and outcome data on enrolled patients for two years. Participants may receive up to $150 dollars for their time.
BESPOKE CRC trial design and patient qualification requirements
You may qualify if all of the following criteria apply
- You are a stage I, II, III or IV colorectal cancer patient.
- Your doctor selected you to receive the Signatera test as part of your routine care.
- You are eligible for post-operative systemic therapy
- You are able to tolerate blood draws from the arm.
- You are 18 years of age or older.

How is the Signatera test performed?
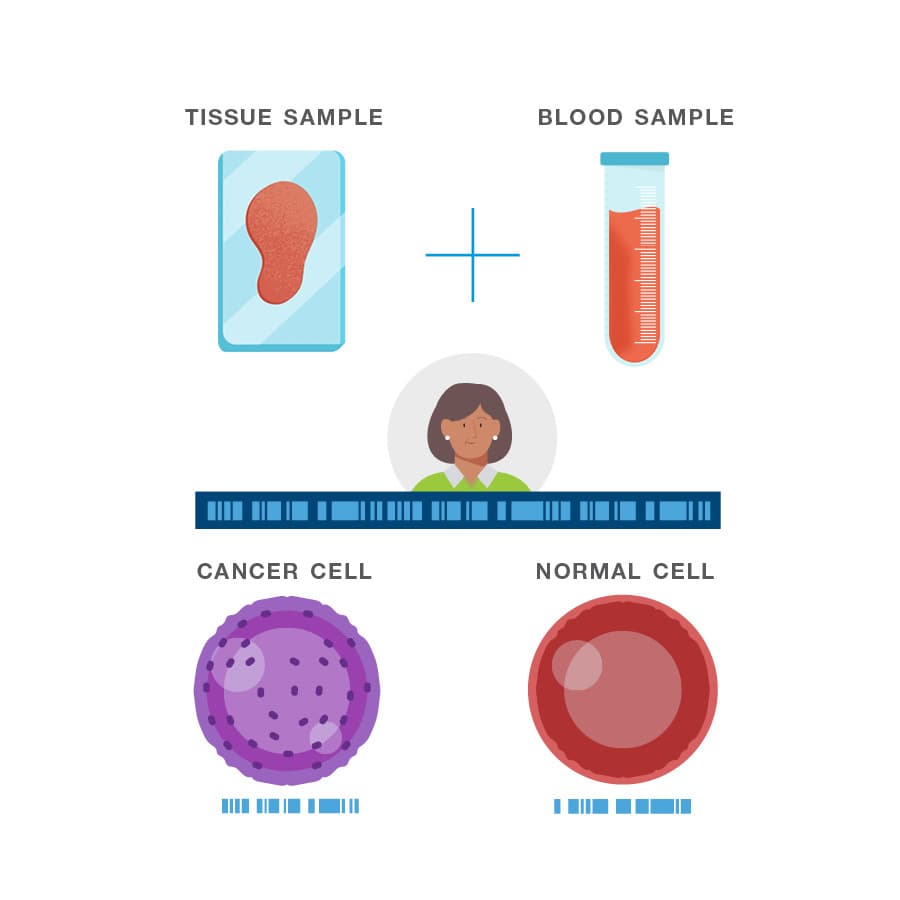
A one-time analysis of both blood and tissue determines your unique set of tumor mutations.

The test is custom-built and personalized for you
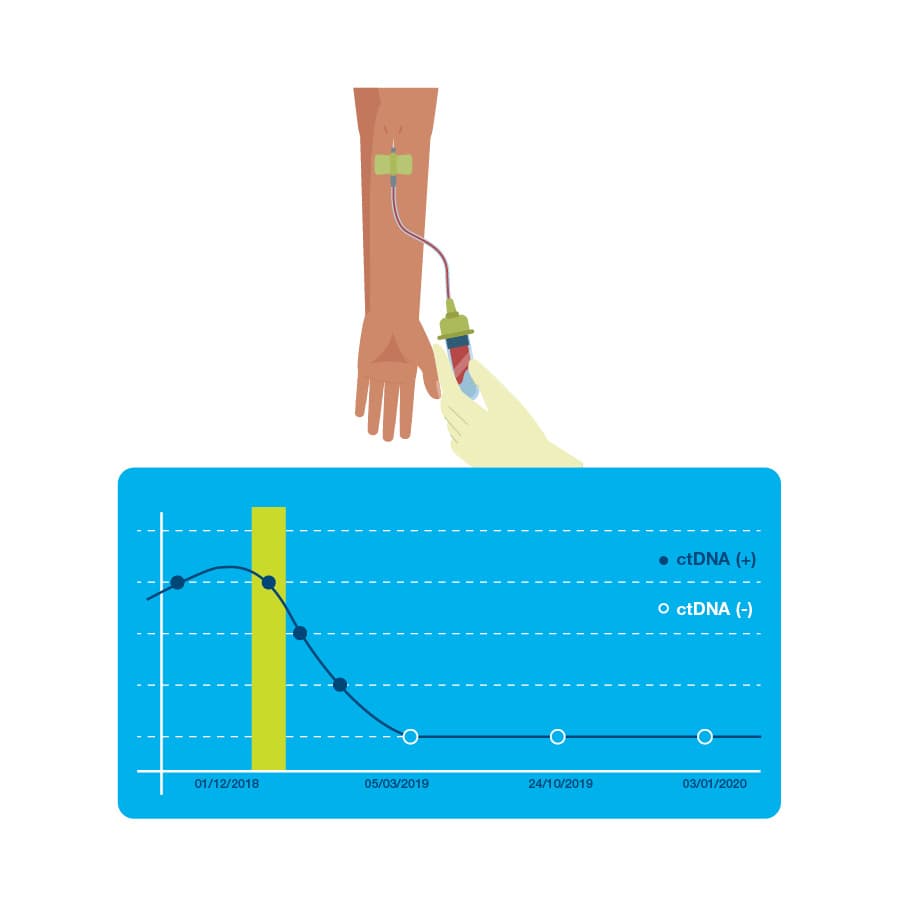
Signatera detects the presence or absence of cancer each time it is ordered as part of your routine follow-up blood tests.
With BESPOKE CRC, expect safety and simplicity.
- You will work with your doctor to set the schedule for blood draws, whether they are in clinic or at home.
- You will be asked to complete questionnaires throughout the two years of the study.
- You will work with your doctor to use Signatera test reports to help guide your care plan including, chemotherapy treatment and additional imaging scans.
