Is MRD testing right for you?
If you’ve been diagnosed with any type of solid tumor cancer, like breast cancer, colorectal cancer, bladder cancer, or ovarian cancer, you probably have many questions like, “Is the cancer still lurking?” or “is the cancer coming back?” or “Is my treatment working?” It is important that you talk with your cancer care team about what tools are available to help answer these important clinical questions, which may make it possible to intervene earlier.
Understanding MRD Testing:
MRD testing, short for Molecular Residual Disease testing or Minimal Residual Disease testing, is a specialized method used to detect tiny amounts of cancer DNA that may be left in the body after treatment. It’s a powerful tool for monitoring cancer and making decisions about further treatment.

How Signatera™ Works: a personalized and tumor informed approach to MRD surveillance
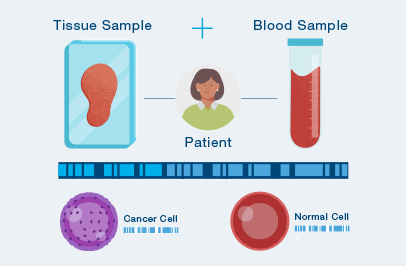
A one-time analysis of both blood and tissue determines your unique set of tumor mutations.

The MRD test is custom-built and personalized just for you, using your own tumor tissue.
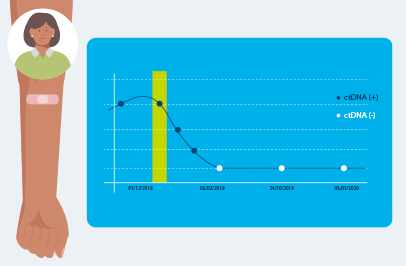
Signatera™ MRD test detects small fragments of ctDNA each time it is ordered as part of your routine follow-up blood tests. Patients who are ctDNA positive by Signatera are more likely to relapse.
Why Signatera™?
Signatera™ is a unique blood test, personalized for each patient using their own tumor tissue. It is used to detect cancer release earlier and treatment response better than standard of care tools.
A doctor may order Signatera™, along with other routine follow up exams to determine:
- If there are any signs of cancer remaining in the body
- If the treatment (ie chemotherapy, radiation, immune checkpoint inhibitors) is working
- If the cancer may be coming back
Signatera™ is effective for many types of cancer

I have colorectal cancer
If you’ve been diagnosed with colorectal cancer:
Discover how Signatera™ MRD testing can help find any remaining cancer after treatment or surgery and/or may help detect a recurrence earlier.

I am on immunotherapy
If you are on immunotherapy:
Explore how Signatera™ MRD testing can help determine if your ICI treatment is working.

I have breast cancer
If you’ve been diagnosed with breast cancer:
Learn how Signatera™ can monitor your response to treatment or detect signs of cancer returning.
What is Signatera™?
Signatera™ is a special type of MRD test that is designed using your own tumor. It’s personalized just for you. Here’s what it can do:
- Identify if there are signs of cancer remaining in the body after surgery or definitive therapy
- Determine if you will benefit from additional therapy after surgery
- Determine if treatment (e.g., chemotherapy, radiation) is working
- Find out if the cancer may be recurring, earlier than standard of care tools
How to have a conversation with your physician about MRD testing
Patients often have questions about their care after a cancer diagnosis or treatment. You should talk to your care team about how to check if your treatment is working and how to watch for signs that the cancer might come back. Signatera™ is a test that looks for tiny bits of tumor DNA in your blood. It’s personalized, using your own tumor tissue.
My doctor ordered Signatera™ to help monitor my treatment journey. What’s Next?
After each time the test is submitted, your oncologist will talk to you about the results. It might take about 3-4 weeks to get the first result, but later tests commonly take only 7-10 days. Depending on your type of cancer, your doctor might use surveillance tools to monitor your cancer treatment and if treatment is working. Signatera™ works best when used regularly over time. Your doctor might also discuss other tests or treatments to keep an eye on your cancer and make sure you’re getting the best care.
It’s important to ask your doctor any questions you have about your test results and how they might impact your treatment plan.
How Signatera™ helps throughout treatment
Living scan to scan can sometimes create anxiety for people with cancer. The question of relapse may be looming in the back of your mind: Did the treatment work? Is the cancer coming back?
Through shared decision making, you can work closely with your cancer care team to incorporate Signatera™ into your treatment plan to provide additional information for confident decision making. Listen to Brooks Bell, colon cancer survivor, tell her story of how she achieved peace of mind between scans using Signatera™.
Empowering survivors through Signatera™
Hear how Trevor is taking a more proactive approach with his health through Signatera™.
Trevor
Living with colorectal cancer
How to read Signatera™ test results?
Your test results will either be positive or negative for the presence of tumor DNA in your blood. Your doctor will receive the test report and will be able to discuss your results and answer questions. These results provide additional insights and may help guide your treatment plan.
IMPORTANT: Negative ctDNA results may change over time. A negative MRD test result doesn’t guarantee that cancer will never be detected in the future. This is why ongoing monitoring with the Signatera™ test, as directed by your doctor, is recommended—for early detection of remaining tumor DNA
Negative result
Positive result
Test Not Performed (TNP)
Results can change over time
Insurance and Medicare Coverage

Each person’s cancer is as unique as their fingerprint. Signatera™ is a custom-designed ctDNA test that is personalized to each patient’s set of tumor mutations and can identify earlier than traditional tools that may indicate cancer is still present. Knowing this information can help you have a more informed discussion with your doctor regarding your treatment journey.
Signatera™ is covered by Medicare for monitoring disease progression, disease recurrence, or relapse for patients with:
- Stage II-IV and oligometastatic colorectal cancer (CRC) in the adjuvant and recurrence monitoring settings
- Muscle invasive bladder cancer (MIBC) in the adjuvant and recurrence monitoring settings
- Stage II-IV breast cancer in the neoadjuvant setting, regardless of subtype
- Stage IIb and higher breast cancer in the adjuvant and recurrence monitoring settings
- Stage II-IV ovarian, fallopian tube, or primary peritoneal cancer in the adjuvant and recurrence monitoring settings
- For monitoring of response the immune-checkpoint inhibitor (ICI) therapy for patients with any solid tumor
Compassionate Care Program

If you are uninsured or is concerned about your ability to pay for Natera genetic testing, you can contact us at (650) 489-9050 option 2 or oncologybilling@natera.com.


