Evaluate Treatment Response Sooner
Predict Response to
Immunotherapy After
Just 2 Cycles
98%
of patients with an increase in ctDNA at the beginning of cycle 3 did not derive an objective response to immunotherapy treatment1
Identify Exceptional Responders
100% OS
in patients who cleared ctDNA for at least one on-treatment time point (median follow-up beyond first clearance of 25.4 months)1
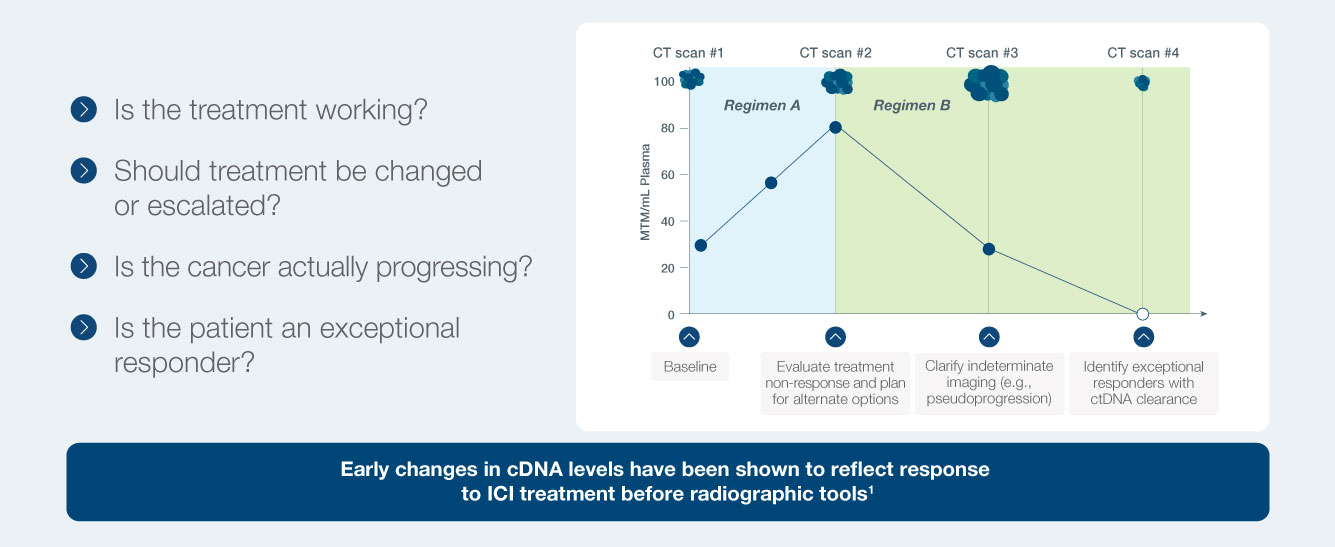
Clarify Indeterminate Imaging
A decrease in ctDNA can precede imaging changes, helping to
identify pseudo-progression
or clarify indeterminate radiologic findings1
Get Started
Longitudinal monitoring with Signatera™ helps answer critical questions
Unlike static tumor markers for cancer, the Signatera™ Molecular Residual Disease Test (MRD) quantifies circulating tumor DNA (ctDNA) over time to provide a real-time assessment of changes in disease burden during immunotherapy (IO) treatment.1
Discover the Treatment Response Monitoring Data
INSPIRE Trial: Predict immunotherapy response across solid tumors
The INSPIRE trial validated Signatera™ ctDNA testing as an early biomarker of immunotherapy response with 98% baseline ctDNA detection across 94 patients and 25 solid tumor types.1
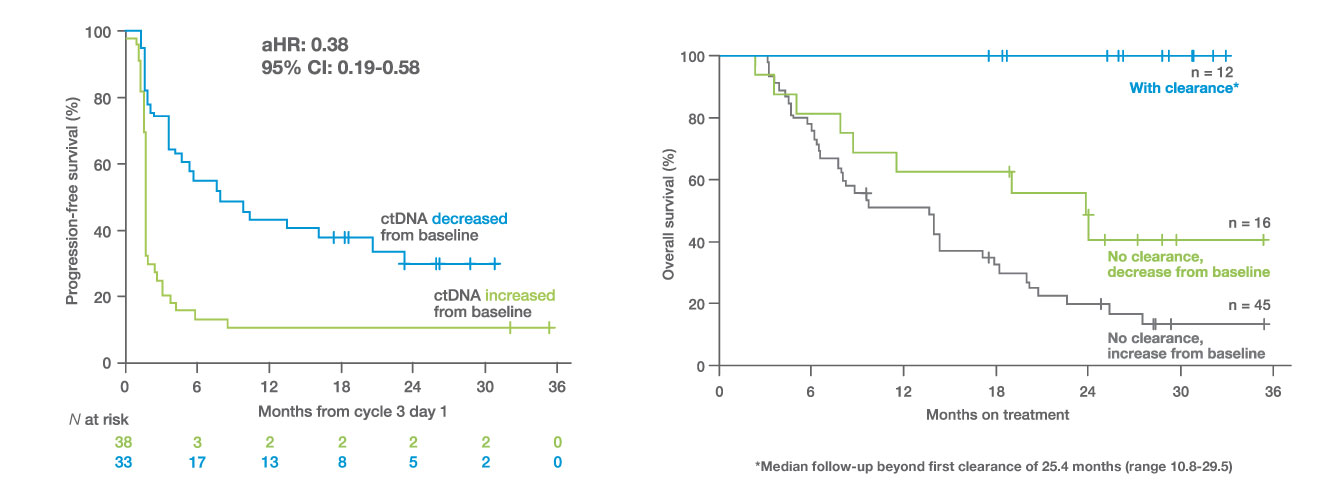
- 98% of patients with an increase in ctDNA at the beginning of cycle 3 did not derive an objective response to immunotherapy treatment
- A decrease in ctDNA from baseline to the beginning of cycle 3 was a strong predictor of increased OS and PFS
- 100% OS in patients who achieved ctDNA clearance at least once during treatment (median follow-up of 25.4 months beyond first clearance)
Melanoma: Inform risk-based treatment selection and monitor immunotherapy response
69 advanced melanoma patients monitored across 555 plasma timepoints to determine if ctDNA can help risk-stratify patients for disease recurrence and predict response to immunotherapy2
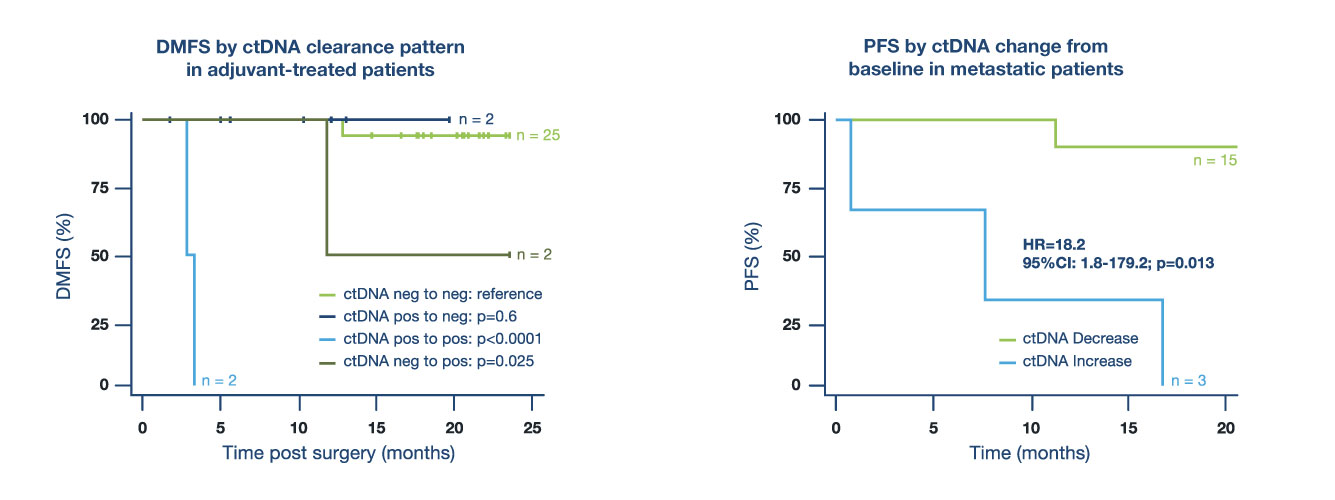
- Patients who were Signatera™ ctDNA positive after resection and before adjuvant therapy had a 10x higher risk of recurrence than ctDNA-negative patients
- Early on-treatment ctDNA dynamics were predictive of PFS in metastatic melanoma patients receiving 1st line ICI treatment
- At week 6, Signatera™ identified that patients with increasing ctDNA had a 18x higher risk of progression than ctDNA-negative patients
NSCLC: Inform early treatment escalation on immunotherapy
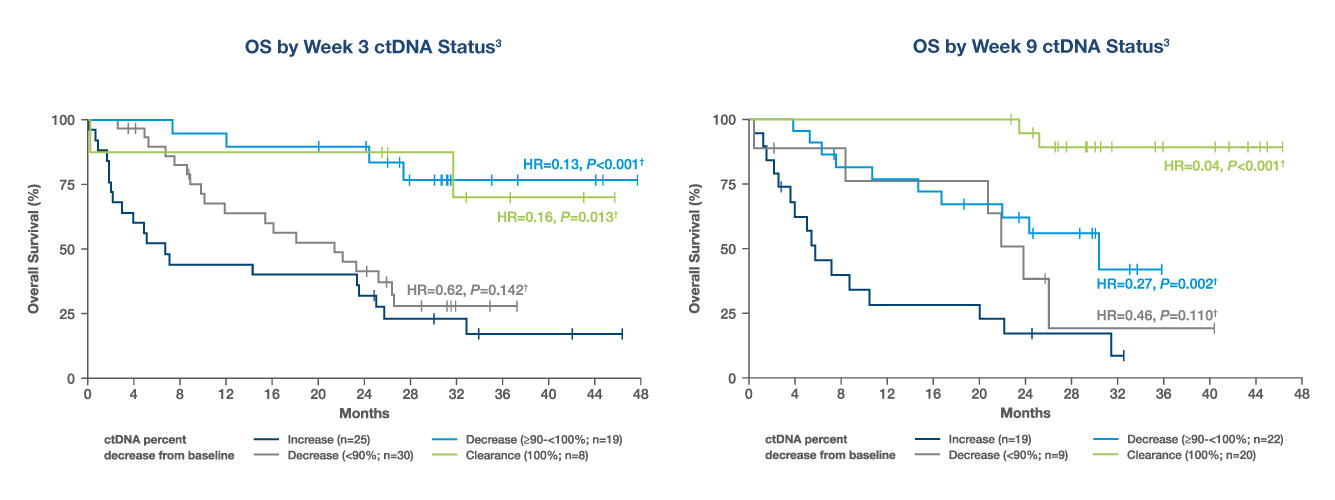
- ctDNA increase was associated with the highest risk of death. ctDNA deep decrease (>90%) and clearance were associated with significantly improved OS
- Composite ctDNA & RECIST assessment may improve prediction of OS benefit from IO
Bladder cancer: Risk-stratify patients after cystectomy and monitor response to immunotherapy
Phase III RCT of atezolizumab vs observation in high-risk MIBC. Exploratory analysis using Signatera™ was performed to determine if ctDNA status after cystectomy can predict who will benefit from adjuvant immunotherapy4
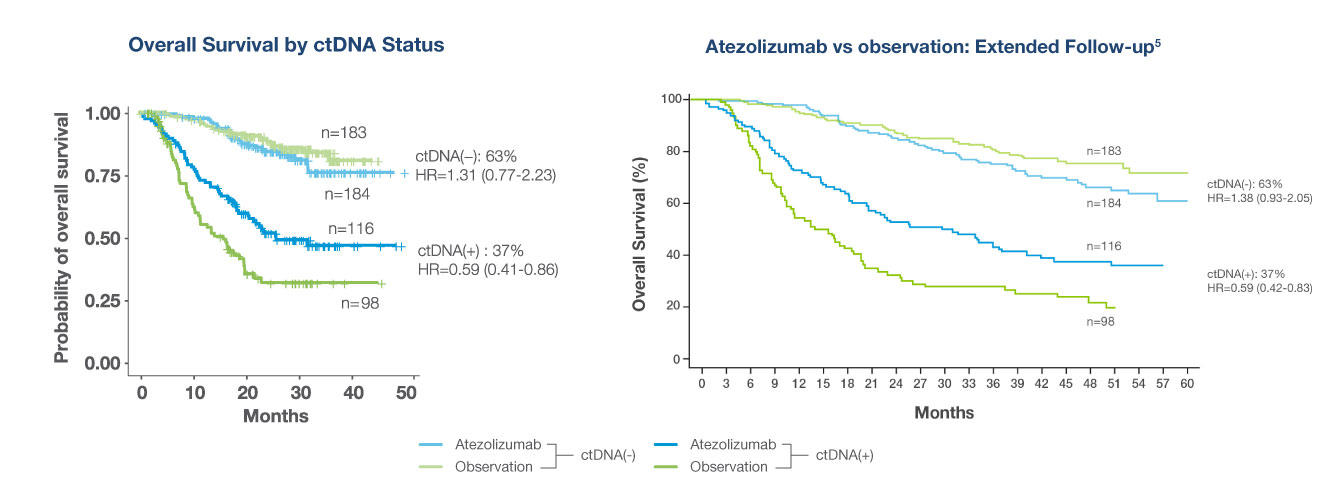
- Signatera™-positivity (ctDNA +) after surgery is predictive of treatment benefit with atezolizumab (OS, HR 0.59)
- ctDNA-negative patients derived no benefit despite treatment with atezolizumab
Watch How Signatera™ Informs Treatment Strategy
Steven Liu, MD
medical oncologist

Do More With Less Tissue
One tumor sample – two tests
Medicare Coverage
- Stage II-IV and oligometastatic colorectal cancer (CRC) in the adjuvant and recurrence monitoring settings
- Muscle invasive bladder cancer (MIBC) in the adjuvant and recurrence monitoring settings
- Stage II-IV breast cancer in the neoadjuvant setting, regardless of subtype
- Stage IIb and higher breast cancer in the adjuvant and recurrence monitoring settings
- Stage I-III non-small cell lung cancer (NSCLC) in the surveillance setting
- Stage II-IV ovarian, fallopian tube, or primary peritoneal cancer in the adjuvant and recurrence monitoring settings
- For monitoring of response to immune-checkpoint inhibitor (ICI) therapy for patients with any solid tumor
Commercial Insurance
We will work with patients so that cost is not a barrier for testing.
We offer an affordable self-pay rate for those patients who do not wish to use insurance.
Learn More

Medicare Coverage
Signatera™ is covered by Medicare for immunotherapy treatment response monitoring across all solid tumor types and stages of cancer.

IO Monitoring Brochure
Learn how Signatera™ can assess immunotherapy response as early as 6 weeks into treatment for patients with solid tumors.1

Patient Case Study
Read how Signatera™ detected a rise in ctDNA levels during immunotherapy, informing the decision to pivot to a combination therapy approach.
Is Signatera™ right for your patients on immunotherapy?
References
1Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1(9):873-881.
2Eroglu Z, Krinshpun S, Kalashnikova E, et al. Circulating tumor DNA based molecular residual disease detection for treatment monitoring in advanced melanoma patients. Cancer (2023). https://doi.org/10.1002/cncr.34716.
3Vokes N, et al. Circulating Tumor DNA (ctDNA) Dynamics and Survival Outcomes in Patients with Advanced NSCLC and High (>50%) PD-L1 Expression, Randomized to Cemiplimab vs Chemotherapy. Presented at ASCO Annual Meeting, Chicago, IL, June 2023.
4Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021.
5Powles T, Assaf ZA, Degaonkar V, et al. Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-invasive Urothelial Carcinoma. European Urology. 2023; https://doi.org/10.1016/j.eururo.2023.06.007.
